electron configuration of cadmium|cadmium orbital diagram : iloilo Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa All Viral Videos Any Queries Or Cross Promotion Please Contact @NinjaKingBot
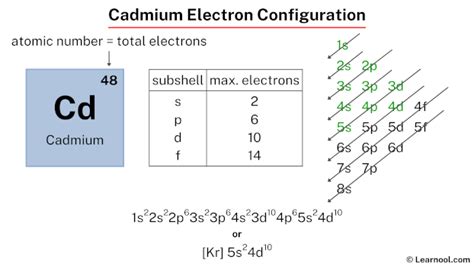
electron configuration of cadmium,The ground-state electron configuration of cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. This electron configuration shows that the last shell of cadmium has two electrons and the d-orbital has a total of ten electrons. Therefore, the valence electronsof cadmium are two. The . Tingnan ang higit paThe total number of electrons in cadmium is forty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cadmium in specific rules in different orbits and orbitals is called the electron . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
The Electron configuration defines the way in which the electrons are structured. electrons located in the atoms of each of the elements of the periodic table. The full .
Electron Configuration of Cadmium; Common Isotopes; Metallic Characteristics; Natural Occurrences; Common Reactions with Cadmium; Common .
electron configuration of cadmium cadmium orbital diagram The cadmium electron configuration, denoted as 5s 2 4d 10 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10, showcases the specific placement of electrons within the atom. This configuration can .
To write the configuration for the Cadmium and the Cadmium ion, first we need to write the electron configuration for just Cadmium (Cd). We first need to find . What is the Electron Configuration of Cadmium? Kr 4d10 5s2 is the electron configuration of Cadmium. How Many Valence Electrons Does Cadmium Have. There are two valence electrons in the outer shell .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . What is the electron configuration of Cd 2 +? Draw the orbital filled diagram for this ion. What is an example of a balanced chemical reaction for the formation of Cadmium hydroxide? Don't forget to include .Electron configuration 4d 10 5s 2: Electrons per shell: 2, 8, 18, 18, 2: Physical properties; Phase at STP: solid: Melting point: 594.22 K (321.07 °C, 609.93 °F) Boiling point: 1040 K (767 °C, 1413 °F) Density (at 20° C) .Atomic Structure of Cadmium. Atomic Radius: 1.71Å; Atomic Volume: 13.1cm 3 /mol; Covalent Radius: 1.48Å; Cross Section (Thermal Neutron Capture)σ a /barns: 2450; .
Protons and Neutrons in Cadmium. Cadmium is a chemical element with atomic number 48 which means there are 48 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x .
Cadmium electron configuration. ← Electronic configurations of elements. Cd (Cadmium) is an element with position number 48 in the periodic table. Located in the V period. Melting point: 321 ℃. Density: 8.64 g/cm 3 . Electronic configuration of the Cadmium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d .The complete electron configuration of Cadmium is 1s2 2s2 2p6 3s2 3p6 4s2 3d104p6 5s2 4d10. Cadmium have 2 valence electrons in its outer shell and its atomic number is 48. The distribution of electrons is as 2 electrons in 1s subshell,2 electrons in 2s subshell, 6 electrons in 2p subshell,2 electrons in 3s,6 electrons in 3p,2 electron in 4s,10 .electron configuration of cadmium This means that a neutral cadmium atom will have a total of 48 electrons surrounding its nucleus. This also tells you that the Cd2+ cation, which has two electrons less than the neutral atom, will have a total of 46 electrons. So, the electron configuration of a neutral cadmium atom looks like this. Cd: 1s22s22p63s23p64s24p64d105s2.Electron configuration 4d 10 5s 2: Electrons per shell: 2, 8, 18, 18, 2: Physical properties; . Cadmium is a chemical element; it has symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two . Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Cadmium (Cd) [Kr] 4d 10 5s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2: 2, 8, 18, 18, 2: 49:
Cadmium -. Cd: properties of free atoms. Cadmium atoms have 48 electrons and the shell structure is 2.8.18.18.2. The ground state electron configuration of ground state gaseous neutral cadmium is [ Kr ]. 4d10. 5s2 and the term symbol is 1S0. Schematic electronic configuration of cadmium. Electron Configuration of Cadmium. Cd: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s2 4d 10 or [Kr] 4d 10 5s 2. Common Isotopes. Cadmium has a total of eight naturally occuring isotopes. These are 106 Cd, 108 Cd, . Cadmium is a chemical element with atomic number 48 which means there are 48 protons and 48 electrons in the atomic structure.The chemical symbol for Cadmium is Cd. Electron Configuration and Oxidation States of Cadmium. Electron configuration of Cadmium is [Kr] 4d10 5s2. Possible oxidation states are +2. Electron .
Full electron configuration of cadmium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. silver ← cadmium → indium. Cadmium, complete electron configuration.

Die Elektronenkonfiguration von Cadmium ist 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. Das chemische Element in Gruppe 12 des Periodensystems heißt Cadmium, seine Ordnungszahl ist 48 und das Symbol dieses Elements ist Cd. Es zeichnet sich dadurch aus, dass es ein bläulich weißes Metall ist, schwer und weich, es ist normalerweise wenig .

La configurazione elettronica completa del cadmio è 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 e la sua versione abbreviata semplificata è [Kr]4d10 5s2. La distribuzione dei 48 elettroni del cadmio è la seguente: Il primo guscio ospita 2 elettroni. Il secondo livello ne ha 8. Nel terzo guscio ci sono 18 elettroni.The electronic configuration of Cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. What is the abbreviated electronic configuration of Cadmium? The abbreviated electronic configuration of Cadmium is [Kr] 4d10 5s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas .
cadmium orbital diagramThe electronic configuration of Cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. What is the abbreviated electronic configuration of Cadmium? The abbreviated electronic configuration of Cadmium is [Kr] 4d10 5s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas .镉的电子排布. 电子配置定义了电子的结构方式。. 电子位于元素周期表中每个元素的原子中。. 镉的全电子构型为 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 其简化简写为 [Kr]4d10 5s2。. 镉的48个电子分布如下:. 第一个壳容纳 2 个电子。. 第二层有8个。. 在第三层壳 .The cadmium orbital notation is a shorthand system designed to represent the exact positions of the electrons in the cadmium atom. This is similar to electron configuration, but numbers are used instead of boxes to represent the positions of the electrons. This orbital notation system always follows the Aufbau principle.
Configuration électronique du cadmium. La configuration électronique définit la manière dont les électrons sont structurés. électrons situés dans les atomes de chacun des éléments du tableau périodique. La configuration électronique complète du cadmium est 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 et sa version abrégée .Chemical element, Cadmium, information from authoritative sources. Look up properties, history, uses, and more. . 2.2 Electron Configuration [Kr]5s 2 4d 10. Los Alamos National Laboratory, U.S. Department of Energy. 2.3 Atomic Radius. Van der Waals Atomic Radius. 158 pm (Van der Waals)
electron configuration of cadmium|cadmium orbital diagram
PH0 · pb 2+ electron configuration
PH1 · electron configuration worksheet answer key
PH2 · electron configuration practice
PH3 · electron configuration for every element
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · cd 2+ orbital diagram
PH7 · cadmium orbital diagram
PH8 · Iba pa